Anti-freeze for winter weather
Funny, but I would have thought that one antifreeze protein would be pretty much like another. I've played with some antifreeze structures before but I never realized that they're more diverse than you might guess. Some antifreeze proteins, like the one from the winter flounder and the sculpin are strictly alpha helical. Antifreeze proteins from yellow meal worms (aka "fish food") however, look pretty rigid and tough, with lots of organized beta sheets and a few random coils.
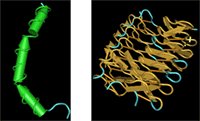
Who would have thought that two protein structures that seem so different would function in a similar way?
Or provide so much entertainment? I found lots of fun things to do with these structures while playing with them in Cn3D.
I noticed that the sequence of one of the structures contained an unusual amount of alanines (a's). I searched for alanines to highlight them in yellow. Which structure seems to be unusually high in alanines?

Coloring the structures by molecule, with the default rendering, shows some interesting yellow bars. Clicking the structure in the region of the bar, highlights a pair of c's in the sequence. Can you tell which structure contains disulfides?
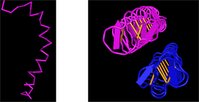
Another question that springs to mind, in looking at the yellow meal worm protein, is this: what holds those two chains together? The two chains look like they're floating in space.
I know that the two chains aren't bound to each other by disulfides.
So, I asked if the two chains were associated by electrostatic interactions (bonds between positive and negatively charged amino acid side chains) by changing the coloring style to reflect charge. This color scheme shows negatively charged amino acids as red, positively charged amino acids as blue, and those with neutral side chains as grey.
You can download a key to the one letter abbreviations and a diagram of the amino acid structures from our web site:
http://www.geospiza.com/education/materials.html
So, are the two chains held together because of interactions between positive and negatively charged sidechains? What do you think the answer is?

I tried something else. I changed the rendering style to space fill and the coloring style to element.
Now:
oxygens are red,
nitrogens, blue;
sulfurs, are yellow (and so are you)
Ah there's nothing like good poetry (and that was nothing like it)
carbons are grey
and hydrogens are white.
I guess that makes everything quite alright.
We don't see hydrogens in X-ray crystal structures, though, since they're not heavy enough to scatter electrons.
Coming back to the two chains, it looks like the carbons fit together like a puzzle (remember, they're grey?).
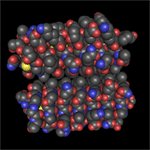
I zoomed in for a better look.

The oxygens are lined up on the inside where the two chains interact. I think they might form hydrogen bonds, but remember, we generally can't see hydrogen bonds in crystal structures.
I guess it's time for NMR and a slice of rhubarb pie.
Subject: Doing biology with bioinformatics
technorati tags: anti-freeze, biochemistry, biology, molecule, nature, proteins





